Innovative Methods for Virtual and Complex Tissue Modeling
On this page:
- Overview
- Virtual Tissue Models
- Complex Tissue Models
- Toxicological Tipping Points
- Helpful Definitions
Overview
Virtual and Complex Tissue Model (VCTM) research is part of a tiered toxicity-testing approach that supports EPA’s chemical hazard assessments while reducing dependency on animal testing. VCTM research aims to address the underlying causes of birth defects and diseases that occur at different life stages.
Innovative methods shed light on how chemical exposures affect the development and function of human organ systems. These methods include virtual, or in silico (computer based), and in vitro “models” that represent human tissues.
- Virtual tissue models use computer software to simulate tissue formation.
- In vitro complex tissue models are built by growing cells in the laboratory under conditions that promote tissue formation.
Adapted from Thomas et al., 2019
Virtual Tissue Models
For an introduction on virtual tissue models, please visit the Virtual and Complex Tissue Models (VCTM) page. To see the most recent VCTM research, view VCTM research publications and presentations.
Examples of computational models used to predict chemical toxicity
- Developmental Toxicity Model: EPA researchers used traditional animal toxicity data and ToxCast high-throughput screening data to build a computational model to study the effects of chemicals on prenatal development. They identified an association between developmental toxicity and key biological signaling pathways that regulate cell growth, cellular differentiation, and inflammation.1
- Blood Vessel Development Model: EPA researchers used ToxCast high-throughput screening data to build a model for predicting potential chemical disruption of blood vessel formation. The model was tested using compounds known to impair vascular development. There was a strong correlation between animal developmental toxicity data and ToxCast data used to positively identify vascular disruptor compounds.2
- Neurovascular Unit Model: The neurovascular unit (NVU) is a group of cells in the brain that interact to form the blood-brain barrier (BBB) during embryonic development and maintain the BBB throughout all lifestages. EPA researchers designed a computational method to screen chemicals for the potential to disrupt NVU development. The novel approach combined the analysis of ToxCast data with data from a set of in vitro assays representing key NVU cell types.3
Examples of toxicokinetic models that link chemical dose to biological effects in target tissues
- Thyroid Model: The thyroid gland, located at the front of the neck, produces hormones that are important for the proper development and function of multiple organs, including the brain. EPA researchers are studying how chemicals impact human brain development by disrupting thyroid function. They are building a computer model that predicts thyroid hormone levels in the developing brain based on data from human thyroid tissue grown in the laboratory.
Examples of cell agent-based models that simulate cellular interactions
Cell agent-based models (ABMs) use computer software to simulate the development of tissues and organs. Cell ABMs developed by EPA researchers and their colleagues comprise the Virtual Embryo™ and involve the production of ‘video clips’ that simulate tissue or organ level responses to chemical exposure. Repeated simulations can be used to investigate the molecular events that lead to birth defects following chemical exposures.
EPA’s Virtual Embryo (v-Embryo™) research develops prediction models to improve our understanding of how chemical exposure may affect unborn children. Researchers integrate new types of in vitro, in vivo, and in silico models to simulate critical steps in fetal development. Virtual Embryo models simulate biological interactions observed during development and predict when chemicals disrupt key biological events in pathways that are thought to lead to adverse effects.

Video clip simulation of palate fusion (Knudsen et al. 2020)
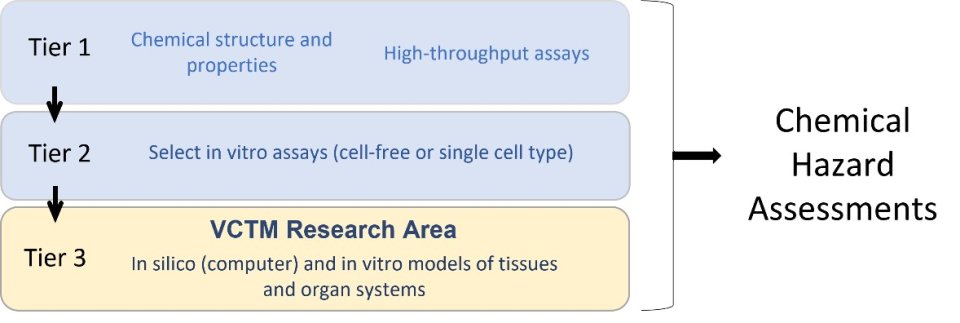
Cell ABM models completed by EPA researchers and their colleagues include developing blood vessels, genital tubercle, limb bud, and palate. Research is underway to model the developing neural tube, axial skeleton, embryonic yolk sac, heart, cornea, and the neurovascular unit (a group of cells that interact to form the blood-brain barrier).
Cell ABM models completed by EPA researchers and their colleagues include developing blood vessels, genital tubercle, limb bud, and palate. Research is underway to model the developing neural tube, axial skeleton, embryonic yolk sac, heart, cornea, and the neurovascular unit (a group of cells that interact to form the blood-brain barrier).
- Blood Vessel Development Model: EPA’s computational modeling to predict Vascular Disruptor Compounds provided the foundation to build a cell ABM model of vascular development.4
- Genital Tubercle Development Model: EPA researchers used ToxCast high-throughput screening data to build a cell ABM model that predicts potential chemical disruption of male genital development. The model simulates changes in development that underly one of the most common birth defects, hypospadias.5
- Palate Fusion Model: EPA researchers and their collaborators used ToxCast high-throughput screening data to develop a cell ABM model that predicts potential chemical disruption of palate fusion during embryonic development. The model simulates changes in development that lead to the birth defect, cleft palate.6
- Neural Tube Model: EPA researchers and their collaborators have mapped the molecular relationships underlying neural tube development. These data set the stage for constructing computer simulations of neural tube closure.7
- Limb-Bud and Skeletal Development Models: EPA researchers are using literature mining tools and ToxCast high-throughput screening data to develop cell ABM models to simulate the effects of a known chemical teratogen, or agent that causes birth defects, on embryonic limb and skeletal formation.8
- Neurovascular Unit (NVU) Model: EPA’s computational modeling to predict NVU disrupting compounds provided the foundation to build a cell agent-based model of the NVU.

Complex Tissue Models
For an introduction on complex tissue models, please visit the Virtual and Complex Tissue Models page.
- Developmental Toxicity Prediction Assay: EPA researchers used a modified embryonic stem cell line to establish an in vitro assay to screen chemicals for potential to disrupt human development.9
- Human Thyroid Organotypic Culture Model: EPA researchers developed an in vitro organotypic culture model using thyroid cells from human donors. When grown under specific conditions in the laboratory, these cells interact to form “microtissues” that look and function like thyroid tissue. The resulting complex tissue model is more representative of human biology than typical in vitro assays. The investigators were then able to apply this model to evaluate ToxCast chemicals for potential thyroid hormone disruption.10
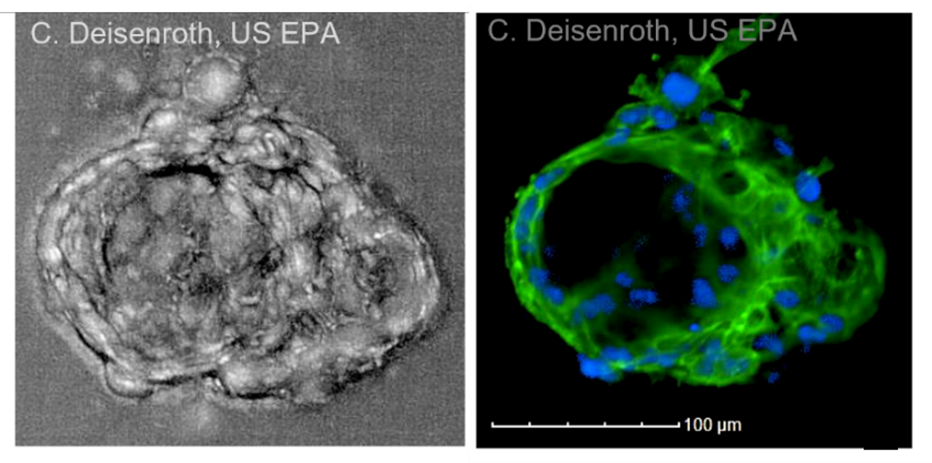
Microscope images of the outside (left) and inside (right) of a mature thyroid microtissue grown in the laboratory. The microtissue shown here is about the size of a grain of table salt. Blue = nucleus; green = cytoskeleton.
- Human Airway Organotypic Culture Models: EPA researchers established an in vitro organotypic culture model that include cell types representative of the respiratory tract. This and other in vitro airway models are being used to test chemicals for toxic effects of inhaled chemicals. The models are also being used to investigate the biological events underlying respiratory toxicity using an adverse outcome pathway approach.11
- Neurovascular Unit (NVU) Organotypic Culture Model: EPA researchers established an in vitro NVU model representing the cell types and tissue structure found during early human brain development. This 3D model will be used as part of a tiered testing strategy to evaluate whether the blood-brain barrier affects the toxicity of potential developmental neurotoxicants.
- Embryonic Heart Development Model: EPA researchers have established an in vitro model that can be used to assess chemical effects under fluid flow conditions representative of early heart development. This model may be used to evaluate chemicals for the potential to cause heart defects.
- Human Pregnancy Models (Placenta): EPA researchers are developing new approach methods to assess human pregnancy physiology and toxicity, including in vitro models of the human placenta.
Toxicological Tipping Points
For an introduction on tipping points, please visit the Virtual and Complex Tissue Models page.
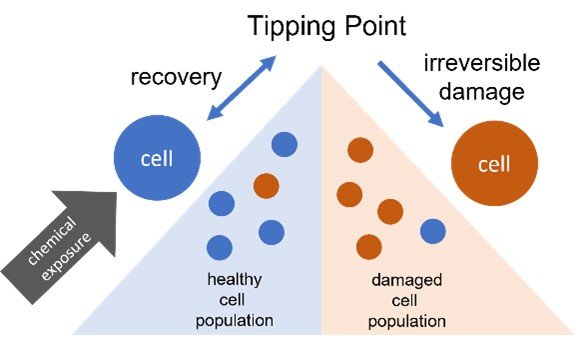
- In vitro Tipping Point Analysis: High-content imaging (HCI) is a high-throughput in vitro assay that measures cellular changes caused by chemical exposures. EPA researchers used HCI to analyze cellular changes in response to chemical exposure to identify the tipping point at which the cells did not show recovery towards a normal state.12
- Developmental Toxicity Tipping Point Analysis: EPA researchers identified a toxicological tipping point at which germ layer specification (an early event of embryonic development) was altered following exposure to a chemical known to cause birth defects.13
Citations
- Predictive models of prenatal developmental toxicity from ToxCast high-throughput screening data (Sipes et al. 2011)
- Environmental Impact on Vascular Development Predicted by High-Throughput Screening (Kleinstreuer et al. 2011)
- A cross-platform approach to characterize and screen potential neurovascular unit toxicants (Zurlinden et al. 2020)
- A Computational Model Predicting Disruption of Blood Vessel Development (Kleinstreuer et al. 2013)
- Computational Modeling and Simulation of Genital Tubercle Development (Leung et al. 2016)
- Computational Model of Secondary Palate Fusion and Disruption (Hutson et al. 2017)
- An ontology for developmental processes and toxicities of neural tube closure (Heusinkveld et al. 2021)
- Computational model for fetal skeletal defects potentially linked to disruption of retinoic acid signaling (Pierro et al. 2022)
- The DevTox Germ Layer Reporter Platform: An Assay Adaptation of the Human Pluripotent Stem Cell Test (Gamble et al. 2022)
- Development of an In Vitro Human Thyroid Microtissue Model for Chemical Screening (Deisenroth et al. 2020)
- Exposure Effects Beyond the Epithelial Barrier: Trans-Epithelial Induction of Oxidative Stress by Diesel Exhaust Particulates in Lung Fibroblasts in an Organotypic Human Airway Model (Faber et al. 2020)
- Using ToxCast data to reconstruct dynamic cell state trajectories and estimate toxicological points of departure (Shah et al. 2016)
- Molecular characterization of a toxicological tipping point during human stem cell differentiation (Saili et al. 2020)
