Sandy, Utah (BD Medical)
BD Medical is located at 9450 South State Street, Sandy, UT. The facility uses ethylene oxide (EtO) to sterilize medical equipment and materials.
EPA scientists and analysts recently completed a risk assessment to understand the impact of EtO emissions from the BD Medical facility. As part of this risk assessment, we used the most recent available information about how much EtO the company emits into the air and we modeled estimated cancer risks to people living nearby. The risk assessment identified elevated cancer risk in the Sandy community. EPA is committed to working with state and local agencies, facilities, and communities to reduce this risk.
BD Medical
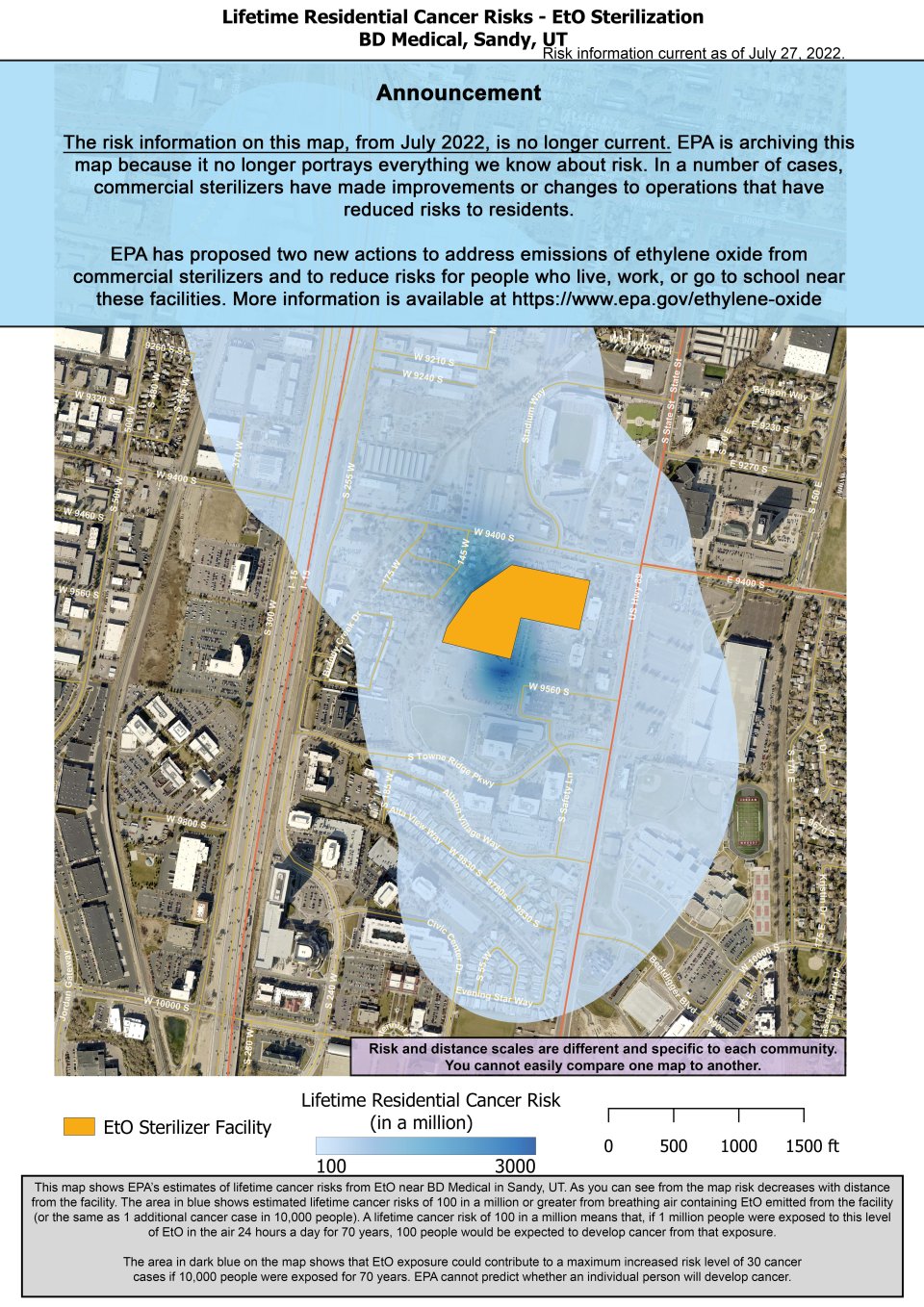
This map shows EPA’s estimates of lifetime cancer risks from EtO near BD Medical in Sandy, UT. As you can see from the map risk decreases with distance from the facility.
The area in blue shows estimated lifetime cancer risks of 100 in a million or greater from breathing air containing EtO emitted from the facility (or the same as 1 additional cancer case in 10,000 people). A lifetime cancer risk of 100 in a million means that, if 1 million people were exposed to this level of EtO in the air 24 hours a day for 70 years, 100 people would be expected to develop cancer from that exposure.
The area in dark blue on the map shows that EtO exposure could contribute to a maximum increased risk level of 30 cancer cases if 10,000 people were exposed for 70 years (or 3,000 in 1 million). EPA cannot predict whether an individual person will develop cancer.
View a larger version of the map and legend in a new browser tab.
For this risk assessment, we looked at excess cancer risk attributable to a single chemical, EtO. This estimated risk is in addition to the risk of developing cancer from other causes. This is a worst-case scenario that assumes a person stays in the highest risk area 24 hours a day continuously for 70 years. EPA takes this approach because we want to be protective of the most exposed and most vulnerable individuals from risk associated with EtO emissions from this facility.
Community Details
BD Medical is a medical technology manufacturing company which operates a sterilization facility at 9450 State St. in Sandy, Utah. The facility has been in operation since 2007 and sterilizes IV catheters and other medical equipment. On October 29, 2021, the Utah Division of Air Quality conducted a compliance inspection of the BD Medical facility in Sandy, UT and determined the source was in compliance with all applicable conditions of the State-issued Approval Order.
What EPA is Doing to Address Ethylene Oxide
Now: EPA is working with the State of Utah to reduce emissions at BD Medical. EPA has provided technical support to our air agency partners as part of this work. The Agency is reviewing controls on regulated equipment and processes that emit EtO to determine whether additional air pollution controls are needed. This review includes examining new developments in practices, processes and control technologies, considering cost and feasibility, as well as addressing any previously unregulated emission points.
EPA has also provided the Utah Department of Environmental Quality (UDEQ) with a Community-Scale Air Toxics Ambient Monitoring grant to evaluate ethylene oxide concentrations near the facility and at other locations in the Salt Lake City area. This effort began earlier this year with a final report expected in the fall of 2023. UDEQ staff will present an update on monitoring activities at the community meeting which will inform efforts to evaluate and reduce ethylene oxide risk in the area surrounding the facility.
BD Medical has announced plans to reduce ethylene oxide emissions from its sterilization facility by 90-95% within the next year. In alignment with the FDA Innovation Challenge, BD Medical is currently installing a new control system to reduce fugitive emissions of ethylene oxide from the facility and is also implementing cycle optimization to reduce total ethylene oxide consumption. The fugitive emissions control system is scheduled to be completed by the end of 2022. The company estimates this upgrade will reduce emissions by 90%.
Soon: Later this year, EPA will propose strengthening current regulations for Ethylene Oxide Commercial Sterilizers. EPA will consider risk as part of the proposed regulations.
- Learn more about regulation for EtO Sterilization Facilities.
- Learn more about actions you can take.
View the Community Meeting
EPA hosted a community meeting for Sandy area residents on October 20, 2022, to present the results of the agency’s 2022 ethylene oxide risk modeling analysis for the BD Medical sterilization facility.
Please click here to view a video recording of the meeting .