Villalba, Puerto Rico (Medtronic PR Operation Co.)
The Medtronic facility located at Carretera 151, Bo. Villalba Arriba in Villalba, PR opened in 1974 and has been conducting sterilization activities since 1998. The facility sterilizes medical devices manufactured on-site including leads and catheters used in pacemakers and pumps used for the treatment of Parkinson’s disease.
EPA scientists and analysts recently completed a risk assessment to understand the impact of EtO emissions from the Medtronic PR Operation Co. facility. As part of this risk assessment, we used the most recent available information about how much EtO the company emits into the air and we modeled estimated cancer risks to people living nearby. The risk assessment identified elevated cancer risk in the Villalba community. EPA is committed to working with state and local agencies, facilities, and communities to reduce this risk.
Medtronic PR Operation Co.
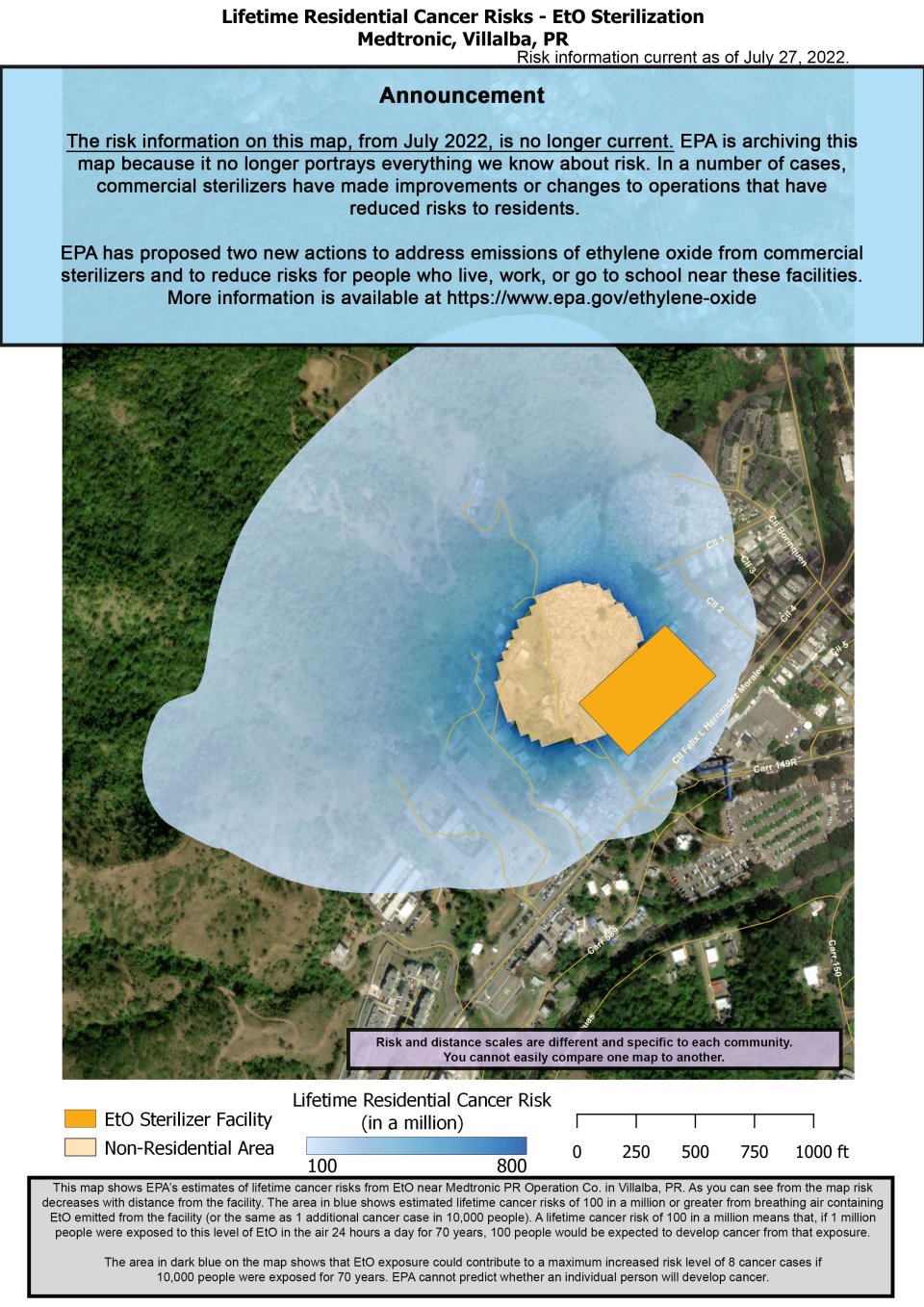
This map shows EPA’s estimates of lifetime cancer risks from EtO near Medtronic PR Operation Co. in Villalba, PR. As you can see from the map risk decreases with distance from the facility.
The area in blue shows estimated lifetime cancer risks of 100 in a million or greater from breathing air containing EtO emitted from the facility (or the same as 1 additional cancer case in 10,000 people). A lifetime cancer risk of 100 in a million means that, if 1 million people were exposed to this level of EtO in the air 24 hours a day for 70 years, 100 people would be expected to develop cancer from that exposure.
The area in dark blue on the map shows that EtO exposure could contribute to a maximum increased risk level of 8 cancer cases if 10,000 people were exposed for 70 years (or 800 in 1 million). EPA cannot predict whether an individual person will develop cancer.
View a larger version of the map and legend in a new browser tab.
For this risk assessment, we looked at excess cancer risk attributable to a single chemical, EtO. This estimated risk is in addition to the risk of developing cancer from other causes. This is a worst-case scenario that assumes a person stays in the highest risk area 24 hours a day continuously for 70 years. EPA takes this approach because we want to be protective of the most exposed and most vulnerable individuals from risk associated with EtO emissions from this facility.
What EPA is Doing to Address Ethylene Oxide
In March 2024, the U.S. Environmental Protection Agency (EPA) strengthened Clean Air Act (CAA) standards for ethylene oxide (EtO) emissions from commercial sterilization facilities. To protect the public and the environment, EPA creates and enforces the rules according to a variety of environmental laws and regulations. The CAA regulates toxic substances in the air and EtO is classified as a toxic substance in the air.
EPA and the Puerto Rico’s Department of Natural and Environmental Resources (DNER) are committed to work together to facilitate and expedite any effort to reduce emissions at Medtronic PR Operations Co.
- Learn more about regulation for EtO Sterilization Facilities.
- For more information about actions you can take.
- Learn more about EPA’s updated EtO standards.
Community Updates
In March 2024, EPA strengthened Clean Air Act (CAA) standards for EtO emissions from commercial sterilizers. Community updates on the Villalba facility and the updated standards are below.
In January 2023, EPA worked with the Puerto Rico Department of Natural and Environmental Resources (PRDNER) and local partners to host a community public meeting for the Villalba community to learn more about EtO and risks from commercial sterilizers.”
January 24, 2023 Community Presentation