In the Field - Sample Handling
Sample Selection
Species Identification
The objective of monitoring studies is to determine the magnitude of contamination in target fish and shellfish species. It is crucial that species are properly identified and that all individuals in a composite sample are the same species.
Species identification should be conducted only by experienced personnel knowledgeable of the taxonomy of species in the waterbodies that are in the contaminant monitoring program. Taxonomic keys should be consulted for species identification.
Initial Inspection and Sorting
All collected species should be inspected carefully to ensure that their skin, fins, shells, carapace, extremities, and exoskeletons have not been damaged or cracked by the sampling equipment. Damaged specimens should be discarded.
Individual fish should be rinsed in ambient water to remove any foreign material from the external surface. Fish should be placed in a live well or euthanized using non-chemical methods.
Individual specimens of each species should be grouped by species and general size class, and the species name and all other appropriate information should be recorded on the field record forms.
Bivalves (oysters, clams, scallops, and mussels) adhering to one another should be separated and scrubbed with a natural fiber brush or nylon brush (if not measuring microplastics) to remove any adhering detritus or fouling organisms from the exterior shell surfaces. After bivalves and crustaceans have been rinsed, individual specimens should be grouped by species.
A few shellfish specimens may be resected (edible portions removed) to determine wet weight of the edible portions. This will provide an estimate of the number of individuals required to ensure that the recommended sample weight (based on the monitoring program design) is attained. The individuals used to determine the wet weight of the edible portion should not be used for target analyte analyses.
Length or Size Measurements
Once the sorting process is complete, nitrile gloves should be worn while handling the organisms. Collected fish and shellfish should satisfy any legal requirements of harvestable size or weight, or at least be of consumable size if no legal harvest requirements are in effect. To be consistent with the convention used by most fisheries biologists in the United States, total length should be measured according to Length or Size Measurements (pdf), and sample measurements and relevant information should be documented (e.g., in a field record form or app).
Each composite sample must consist of all the same species and individual fish should be of similar size (i.e., all within 75% of the length of the largest fish). The same species fish can be lined up on aluminum foil in order of size and then grouped until there are visually matched groups. The sample (individual or composite) must provide sufficient grams of fish tissue for chemical analyses, based on the total mass requirements for chemical analyses allowing for some duplicate analysis as well.
Morphological Abnormalities
In general, people avoid consuming fish with obvious external anomalies and if possible, fish with morphological abnormalities should not be included in the analysis. If gross morphological conditions on fish such as deformities, fin erosion, open sores and tumors are detected on rejected fish, it may be useful to your fish program to document what was observed on rejected fish.
Observations of morphological abnormalities must be documented if the fish tissue is to be analyzed. Fin erosion may be related to pollution, but it is also a common phenomenon of spawning anadromous fish. Open sores and tumors on fish may be related to pollution. The presence of parasites should also be recorded because they can impact sample integrity, indicate reduced fitness of the fish, and could reduce the body burden of some contaminants.
Sex Determination (Optional)
An experienced fisheries biologist can often make a preliminary sex determination of the collected fish and crustacean species by visual inspection. The bodies of collected species specimens should not be dissected or shucked in the field to determine sex. Sex can be determined through internal examination of the gonads during laboratory processing. Sex cannot be determined in the field for bivalve mollusks. They must be shucked and gonadal material examined with a microscope.
Sample Packaging
After initial processing to determine species, size, morphological abnormalities, and sex (optional), each specimen should be packaged. There are different packaging methods that should be considered depending on the objectives of the monitoring program.
Fish
- Wrap each fish in aluminum foil with the dull side in contact with the fish. This can prevent spines from poking through plastic bags. For specimens with sharp fins, spines may be broken (via gloved hands) to prevent perforation of wrapping materials. The broken section of the fins should be included with the fish sample. Aluminum foil should not be used if aluminum analysis is to be performed.
- Each individual fish should be placed in a waterproof plastic bag and sealed. Specimens for a composite can all then be placed in another bag together. This procedure allows uniformity in the thawing processes; fish aren’t frozen together in one lump.
- The sample identification label should be attached to the surface of the outermost plastic bag. The label should include the sample ID, project name, site identification, date, common name, and specimen length.
- A chain of custody tag or label may be attached to the outside of the plastic bag with clear tape; however, one chain of custody tag or label per shipment is sufficient.
- All packaged individual specimens in a composite sample should be kept together (if possible) in one large waterproof plastic bag in the same shipping container (ice chest) for transport. The label should include the common name and specimen length range.
- Once packaged, samples should be cooled on dry ice for shipment or placed in freezers as soon as possible. If samples will be transported to a laboratory or other facility to be frozen before shipment, wet ice can be used to transport fish samples in the coolers to the laboratory or facility.
Shellfish
- Shellfish specimens should be wrapped individually in aluminum foil. Some crustacean species have sharp spines on their carapace that might puncture the aluminum foil wrapping. One of these procedures should be done to reduce punctures: double-wrap the entire specimen in extra heavy-duty aluminum foil, place clean cork stoppers over protruding spines prior to wrapping in aluminum foil, or wrap the spines with multiple layers of foil before wrapping the entire specimen.
- Because of the generally smaller size of shellfish, several wrapped shellfish specimens (within the same composite sample) may be placed in the same waterproof plastic bag.
- A chain of custody label or tag should be completed for each shellfish composite sample and attached to the outside of the plastic bag. Record appropriate information on the field record sheet and chain of custody form.
- Once packaged, samples should be cooled on dry ice for shipment or placed in freezers as soon as possible. If samples will be transported to a laboratory or other facility to be frozen before shipment, wet ice can be used to transport shellfish samples in the coolers to the laboratory or facility.
Sample Preservation and Shipping
Wet ice could be used in the field if the samples are being placed in a freezer within 24 hour of sample collection.
Dry ice is recommended for all shipments and is always needed if the time frame is greater than 24 hours. Fish samples should be packed on sufficient dry ice to keep them frozen for up to 48 hours. For the national fish studies, the EPA recommends 50 pounds of dry ice per 70-quart cooler to protect the integrity of the sample during shipment. Do not use dry ice pellets for shipping because pellets do not last long. Another option is to add fish samples to a freezer capable of maintaining temperatures of ≤-20°C within 24 hours of collection and store the frozen samples until shipment within two weeks of sample collection. If fish samples cannot be stored in a freezer within 24 hours of collection, the field crew should replenish the supply of dry ice in the cooler containing the samples, at least daily, until the samples can be properly frozen or shipped. When shipping frozen samples, use a foil-backed dry ice liner and foam pad inside the cooler.
When shipping with dry ice, layer the dry ice and samples to increase contact between samples and dry ice. Each container should be secured with packaging tape and sealed with a chain-of-custody label which contains the date and signature of the sampler. The seal should be affixed such that the shipping container cannot be opened without breaking the seal to protect and document the integrity of the contents from field to laboratory. The fish and shellfish samples should be hand-delivered or shipped overnight to the processing laboratory as soon as possible after collection. Shipments need to be tracked since it is not unusual for shipments of multiple large coolers to not arrive the day after sampling. The time the samples were collected and time of their arrival at the processing laboratory should be recorded on the chain of custody form.
Dry ice should not be transported inside a closed vehicle. Ventilation is essential since the sublimation to gaseous carbon dioxide could be dangerous. Dry ice requires special packaging precautions before shipping by aircraft to comply with U.S. Department of Transportation (DOT) regulations. The Code of Federal Regulations (49 CFR 173.217) classifies dry ice as Hazard Class 9 UN1845 (Hazardous Material). These regulations specify the amount of dry ice that may be shipped by air transport and the type of packaging required. For each shipment by air exceeding 5.5 pounds of dry ice per package, advance arrangements must be made with the carrier. The package must be marked with the net mass and "Carbon Dioxide, Solid" or "Dry Ice" with a statement indicating that the material being refrigerated is to be used for diagnostic or treatment purposes (e.g., frozen tissue samples).
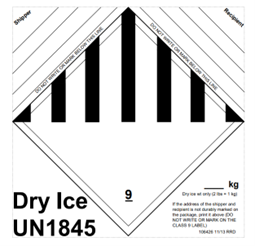
Not more than 200 kg of dry ice may be transported in any one cargo compartment on any aircraft unless the shipper has made special written arrangements with the aircraft operator. Dry ice should have written documentation containing the following information: proper shipping name (Dry ice or Carbon dioxide, solid), Class 9 Dangerous Goods label, UN number 1845, the number of packages, and the net quantity of dry ice in each package. The information must be included with the description of the materials.
The regulations further specify that the packaging must be designed and constructed to permit the release of carbon dioxide gas to prevent a buildup of pressure that could rupture the package. If samples are transported in a cooler, the cooler should have a built-in vent or else vent holes should be drilled into the top of the vertical sides to allow carbon dioxide gas to escape. When the samples are packaged, care should be taken to keep these vents open to prevent the buildup of pressure.
Dry ice availability should be determined in advance and multiple options available to adjust for possible dry ice supply issues. If unavailable, the samples should be transported on wet ice to a freezer and held until dry ice is available for shipping. In addition, a member of the field collection staff should contact the processing laboratory to alert them to the anticipated delivery time of the samples and the name and address of the carrier to be used.
Field collection staff should avoid shipping samples for weekend delivery to the processing laboratory unless prior plans for such a delivery have been agreed upon with the processing laboratory staff. Many programs ship only Monday through Wednesday so that if there are shipping delays, the laboratory will still receive the samples before the weekend.
