EPA EcoBox Tools by Stressors - Chemical
Overview

Chemical stressors may include:
- Organics—containing carbon (e.g., dioxins, furans, PCBs, flame retardants, hydrocarbons, perfluorinated compounds (PFCs), some pharmaceuticals, phthalates, volatiles, semi-volatiles (e.g., polycyclic aromatic hydrocarbons [PAHs]).
- Metals and other inorganics (including nutrients such as calcium, nitrogen, phosphorous)—generally do not contain carbon. For ecological receptors, heavy metals such as lead and mercury and nutrient pollution are a major focus.
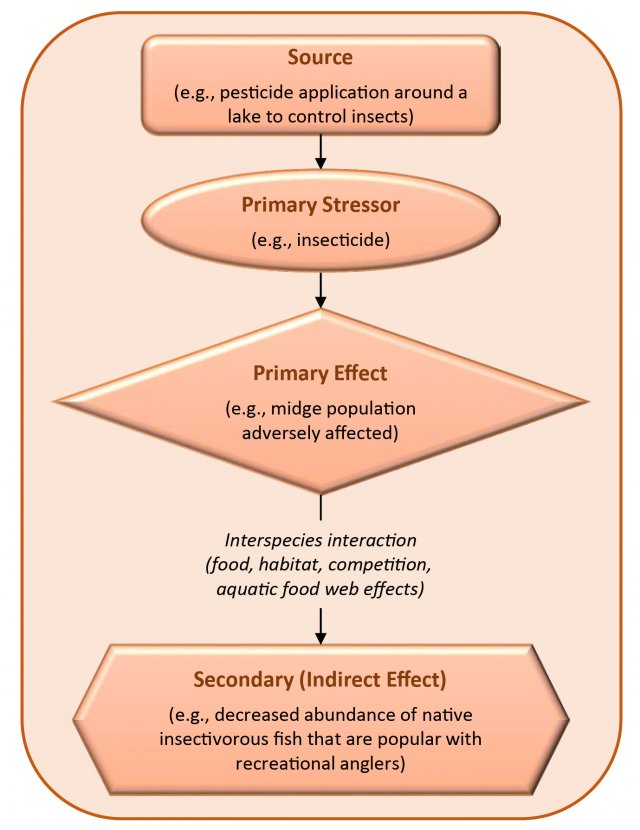
- Pesticides—can be organic compounds (e.g., pyrethroids and paradichlorobenzene) as well as metals and other inorganic substances (e.g., tin compounds historically used as antifoulants).
- Emerging contaminants of concern—may include nanoparticles or nanomaterials (e.g., nanoscale titanium dioxide, nanoscale silver).
Chemical stressors may have natural counterparts or multiple sources. Many chemicals occur naturally (e.g., most metals), are generally widespread from other sources (e.g., PAHs in urban ecosystems), or may have significant sources outside the boundaries of a site (e.g., atmospheric nitrogen). For chemicals that are secondary stressors, an evaluation might focus on metabolites, biodegradation products, or chemicals formed through abiotic processes (U.S. EPA, 1998).
Nutrients (e.g., nitrogen, phosphorous, potassium) are essential to health for animals, plants, and microorganisms. In excess, any nutrient can be harmful. For some species, metals that are micronutrients (e.g., iron, cobalt, magnesium) can be toxic at higher levels. Nitrogen and phosphorus are elements that occur naturally in aquatic ecosystems and support the growth of algae and aquatic plants. On the other hand, eutrophication (nutrient over-enrichment) in aquatic systems, particularly due to excess nitrogen and phosphorous contamination, is a major water quality issue. Secondary stressors can also be formed through ecosystem processes. For example, nutrient inputs into an estuary can decrease dissolved oxygen concentrations (a secondary, indirect impact) because they increase primary production and subsequent decomposition (U.S. EPA, 1998).
General Tools
Resources that are applicable to conducting ERAs for chemical stressors are provided below.
Fate and Transport Considerations for Chemical Stressors
Fate and transport processes “link” the release of chemical stressors at a source with the resultant environmental concentrations to which receptors can be exposed. When a contaminant is released from a source, it is subject to transportMovement within a medium or between media. and transformationChange in a chemical or physical state. in the environment. Compounds can also transferProcess by which chemicals are taken up by a plant or animal either directly from exposure to a contaminated medium (soil, sediment, water) or by eating food containing the chemical. from an environmental medium to biota, a process referred to as bioconcentration or bioaccumulation. Some chemicals can biomagnifyBioaccumulation is the general term describing a process by which chemicals are taken up by a plant or animal either directly from exposure to a contaminated medium (soil, sediment, water) or by eating food containing the chemical. Related terms are bioconcentration which chemicals are absorbed by an animal or plant to levels higher than the surrounding environment; and biomagnification, in which chemical levels in plants or animals increase from transfer through the food web (e.g., predators have greater concentrations of a particular chemical than their prey). as they move up the food chainA food chain is formed as one organism eats another. A food web is a system of interlocking and interdependent food chains, in which each organism supplies energy to another life form. (see the Food Chain module of the Exposure Pathways (Media) Tool Set in EPA EcoBox).
Uptake is the process by which a substance crosses an absorption barrier and is absorbed into the receptor (U.S. EPA, 2011). Uptake is a function of the stressor (e.g., a chemical’s form or a pathogen’s size), the medium (e.g., sorptive properties, presence of solvents), the biological membrane (integrity, permeability), and the organism (sickness, active uptake) (Suter et al., 1994).
Most stressor-response relationships express the amount of stressor in terms of media concentration or potential doseThe amount of a chemical contained in material ingested, air breathed, or bulk material applied to skin. rather than internal doseThe amount of an agent that enters a target by crossing an exposure surface that acts as an absorption barrier.; this limits the utility of uptake estimates in risk calculations. However, biomarkersA contaminant-induced physiological, biochemical, or histological response of an organism. and tissue residues can provide valuable confirmatory evidence that exposure has occurred, and tissue residues in prey organisms can be used for estimating risks to their predators (U.S. EPA, 1998).
See the Exposure Pathways (Media) Tool Set for discussions of bioaccumulation factorsThe ratio between the concentration of a chemical measured in an organism and the concentration of the same chemical in water. (BAFs) and bioconcentration factorRatio of the concentration of a chemical in an organism to the concentration of the chemical in its surrounding aqueous environment. BCF values are surrogate measures of the bioaccumulation potential of a chemical in organisms in the environment. (BCFs) that are used to characterize relationships between the concentrations of chemical stressors in environmental media and concentrations in plant or animal receptors.
| Migration Process | Examples of Fate and Transport Processes |
|---|---|
| Transport |
Within medium
Between media
|
| Transformation |
Chemical change
Physical change
|
|
Transfer – |
|
Important factors that can impact the migration processes described above include the following.
- Certain physicochemical properties of the stressor—for example, half-lifeThe length of time required for the mass, concentration, or activity of a chemical or physical agent to be reduced by one-half., vapor pressure, Henry’s law constant (KH), water solubility, lipophilicity, octanol/water partition coefficient (Kow), solid/water distribution ratio (Kd), organic carbon/water partition coefficient (Koc), octanol/air partition coefficient (Koa).
- Characteristics of the stressor’s environment—for example, surface water flow rate; water hardness; levels of dissolved oxygen in water; temperature; pH; salinity; meteorological factors such as sunlight and precipitation; soil characteristics such as texture (percent sand, silt, and clay), structure (arrangement of the soil particles), and pore space; percent organic matter.
- Chemical class—whether the chemical is organic, inorganic, or a persistent, bioaccumulative, and toxic (PBT) chemical. PBTs are organic or inorganic chemicals that are persistent in the environment, bioaccumulateBioaccumulation is the general term describing a process by which chemicals are taken up by a plant or animal either directly from exposure to a contaminated medium (soil, sediment, water) or by eating food containing the chemical. Related terms are bioconcentration which chemicals are absorbed by an animal or plant to levels higher than the surrounding environment; and biomagnification, in which chemical levels in plants or animals increase from transfer through the food web (e.g., predators have greater concentrations of a particular chemical than their prey). in food chainsA food chain is formed as one organism eats another. A food web is a system of interlocking and interdependent food chains, in which each organism supplies energy to another life form., and are toxic to terrestrial and aquatic systems.
Fate and Transport Tools
There are a number of sources of information related to the environmental fate and transport of contaminants provided in the table below.