Value of Information (VOI) Case Study on ETAP
EPA conducted a value of information (VOI) analysis to weigh the public health and economic trade-offs associated with the timeliness, uncertainty, and costs of conducting the EPA Transcriptomics Assessment Product (ETAP) compared to traditional human health assessment processes. Fewer than a quarter of the tens of thousands of chemicals in commerce--as well as those found in the environment, various waste streams, and the human body--have traditional toxicity or epidemiological data that can inform human health risk assessments. ETAP is a novel human health assessment approach aiming to address this challenge by targeting chemicals that lack traditional toxicity testing data.
VOI Analysis of ETAP
VOI analysis was a recommendation from the 2009 National Academies of Sciences (NAS) report Science and Decisions Advancing Risk Assessment to provide EPA with an objective decision framework to assess the trade-offs of time, uncertainty, and cost in choosing testing methods for a variety of chemical exposure scenarios and decision contexts.
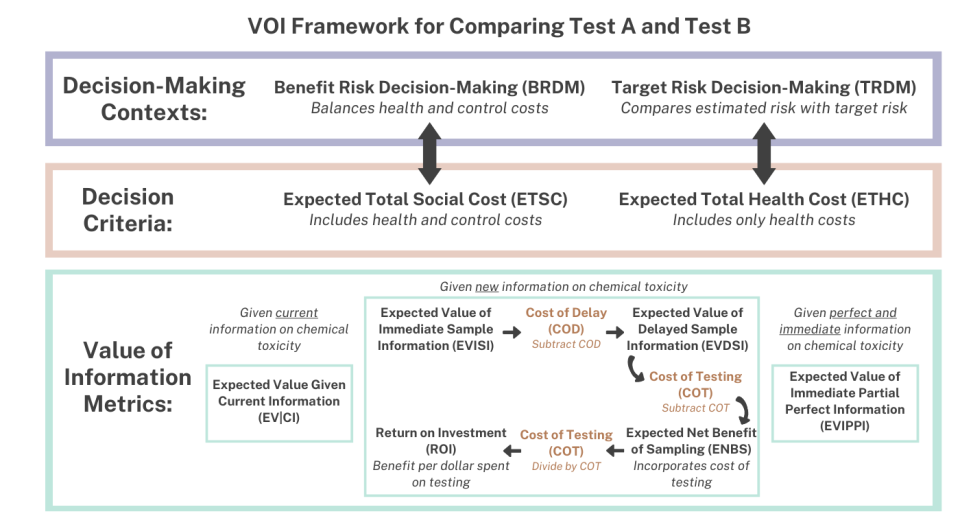
EPA developed and published a VOI framework. This framework was applied to a case study evaluating the human health and economic trade-offs of the recently released ETAP that relies on a standardized five-day in vivo transcriptomic-based toxicity study compared to a traditional human health assessment using a two-year rodent study. Both the ETAP and a traditional human health assessment results in a reference value representing the daily dose of a chemical substance that would be unlikely to result in an adverse health effect following oral exposure in humans.
The process to conduct an ETAP is shorter and less costly, but with the trade-off of presumed greater uncertainty. Once the chemical of interest is in the lab, an ETAP can be completed in less than a year. Traditional toxicity testing and assessment processes can take several years. An analysis of the scientific literature and internal studies indicate high concordance between the ETAP-derived values and traditional animal studies across species, sex, route of exposure, physical chemical properties, toxicokinetic half-life, and technology platform.
In the case study, the ETAP and traditional toxicity testing and human health assessment processes are evaluated using a socioeconomic analysis that compares and quantifies the public health (i.e., societal health benefits) and return on investment associated with obtaining information to inform a regulatory decision.
This video provides more details about the VOI.
Results
In the VOI analysis, the ETAP was the preferred testing method in the majority of scenarios considered. The analysis was performed for various combinations of exposure conditions, health endpoints, exposure control costs, population characteristics, and decision types using a range of values informed by real-world data. The VOI results indicate that reducing the time to decision, realized by using the shorter-duration ETAP process, has significant public health and economic benefits when compared with the longer traditional toxicity testing and human health assessment process.
Final VOI Report
VOI Materials Prepared for BOSC Review
- VOI Case Study Report for BOSC Review (pdf) (3.1 MB)
- VOI Case Study Supplemental Material for BOSC Review (pdf) (1.4 MB)
- Board of Scientific Counselors Panel for VOI
Learn more:
EPA’s Safer Chemicals Research aims to address the challenge of needing more chemical information to make informed, risk-based decisions.
